False: All atoms of the same element contain the same number of protons True or False: Protons have no electrical charge. False: Neutrons have no electrical charge. Atoms of the same element have the same atomic number so the number of protons (and electrons) are same. But they have same or different mass number (like that for isotopes). Different mass number will have different number of neutrons. Answer verified by Toppr. Atoms of the same element can have different properties. True False 2 See answers duttasandipa519 duttasandipa519 Answer: generally it realy does'nt happen so it better to write FALSE. Download coretech laptops & desktops driver. Anshu201988 anshu201988 if each element ha've positive sign it will lose electron e.g = na+ ( sodium) have 12 neutron. 11 electron and 12 proton.
- All Atoms Of An Element Have The Same What
- List Of All Atoms
- All Atoms Of An Element Have The Same Number Of Protons
Many people might think atoms and elements are the same. However, atoms and elements do have a few differences when you start breaking it down. The main difference is elements are made of atoms. Learn other differences between atoms and elements by dissecting these two terms. Explore examples of elements and atoms.
All Atoms Of An Element Have The Same What
What Is an Atom?

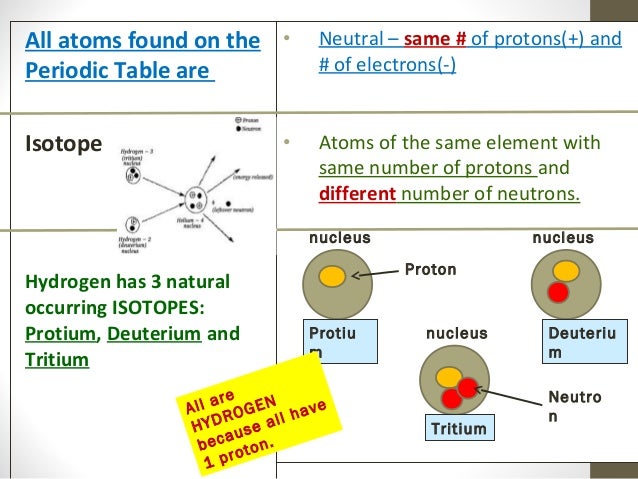
Oct 29, 2015 Because atoms of an element has, essentially, the same number of protons. On the other hand, atomic mass is the sum of total number of protons and neutrons present in an atom. For example, atomic mass of nitrogen is 14 that is, it contains 7 protons and 7 neutrons. Thus, we can conclude that all atoms of the same element must have the same number of protons.
You might have heard the term atom thrown around in chemistry. And, there's a good reason for that; it’s extremely important. Every compound, molecule, or element you come across will be made of atoms. For example, humans are made of atoms. Air is made of atoms. Your computer… made of atoms. Everything is made of atoms.
This is why you can’t begin to understand the difference between atoms and elements without first understanding what an atom is and what it’s made of. Simply put, atoms are the building blocks of elements. They are some of the smallest bits of what you would call ordinary matter.
Structure of an Atom
Like everything else, atoms have a few different things floating around inside of them. These subatomic particles include:
- neutrons - with no charge
- protons - positively charged
- electrons - negatively charged
The protons and neutrons are in the atomic nucleus of the atom, while the electrons orbit the atomic nucleus. Think of this as similar to the way the planets all orbit the sun. That’s an atom in a nutshell. Well, if a nutshell was orbited by lots of little electrons, that is.
What Is an Element?
With atoms out of the way, it’s time to look at elements. If you’ve ever seen a Periodic Table of Elements, then you probably have some idea of what elements are. But to break it down into a simple definition, elements are all the different types of atoms we know exist on Earth. They are arranged by their atomic number on the Periodic Table of Elements.
For example, gold is an element. If you were to hold a chunk of pure gold in your hand, you would be holding an element. Other elements include:
- Hydrogen
- Boron
- Carbon
- Neon
- Magnesium
- Silicon
- Aluminum
- Chloride
- Oxygen
- Calcium
Elements and Atomic Number
List Of All Atoms
What makes something an element is the fact that all the atoms have the same number of protons in the nucleus. While you can find them all on the periodic table, let’s look at the common elements’ mercury and copper.
- Mercury is an element with 80 protons in its nucleus. It has an atomic number of 80.
- Copper is made of atoms with 29 protons in the nucleus. Therefore, it has an atomic number of 29.
What Is the Difference Between an Element and a Molecule?
With atoms and elements all cleared up, it’s important to understand the difference between a molecule and an element. Because like all things in the world, elements and molecules are both made of atoms. You know elements are all the different types of atoms on the periodic table. Molecules are what you get when those atoms are combined. Unlike elements, molecules can be made from the same or different elements. The key to a molecule is that two or more atoms are bonded together.
All Atoms Of An Element Have The Same Number Of Protons
For example, water is a molecule made of hydrogen and oxygen. It’s actually made of two hydrogen atoms and one oxygen atom. You can also have molecules of a single atom bonded together like two oxygen atoms. This makes up the oxygen humans breathe.
Difference Between Atoms and Elements
It can be easy to see why elements and atoms get confused because elements are atoms. They are just a group of all the same kind of atoms. All the known elements on Earth can be found in the periodic table of elements.
Science is fun right? Keep the chemistry fun going by exploring the difference between atoms and molecules. You can also have more chemistry fun by reading up on molecules and compounds.
Certified Teacher
