In order to write the Sulfur electron configuration we first need to know the number of electrons for the S atom (there are 16 electrons). When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the Sulfur atom. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Find the Atomic Number. To find out the atomic number of sulfur, we can use the periodic table.
How Many Electrons Are In Sulfur
What are the four quantum numbers for sulfur?
1 Answer
Explanation:
Sulfur = element #16

The first quantum number tells you which energy level electron #16 is in, it is in level 3
The second number tells you which sublevel the electron is in - it is in the 3p sublevel. We use s=0, p=1, d=2, f=3
The third quantum number tells you which orbital of the sublevel an electron is in. The three orbitals of the 3p sublevel will each fill with an up spin electron first, the left most orbital will get also down spin electron. This is electron #16, so its third quantum number is -1. The three orbitals for a p sublevel are labeled -1, 0, +1
The final number tells you the spin - use
Here is a video to help more with quantum numbers:
Video from: Noel Pauller
Hope this helps!
Related questions
How many valence electrons can Sulfur hold?
Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. So in addition to being octet, sulfur can expand octet to have 10 or 12 electrons.
Can sulfur have more than 8 valence electrons?
Unlike atoms from periods one and two that only have the s and p orbitals (total of 8 valence electrons), atoms like phosphorus, sulfur, and chlorine can have more than 8 electrons because they are not restricted to the s and p orbitals and have a d orbital for additional electrons needed for bonding.
Does sulfur have 16 valence electrons?
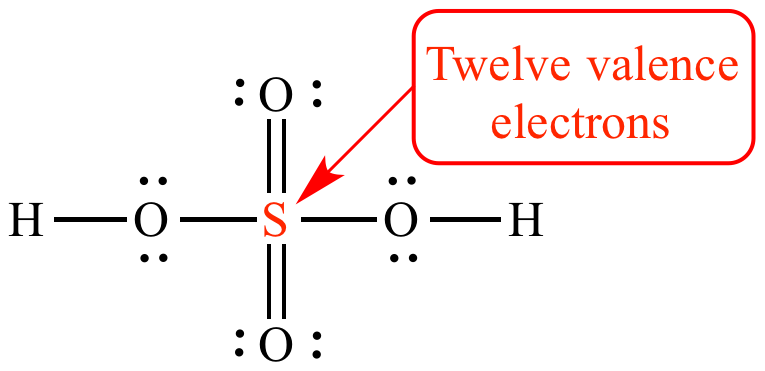
Sulfur has 6 valence electrons located in its outermost orbit. When looking at the sulfur atom, it contains 16 total electrons.
Does sulfur have 7 valence electrons?
Explanation: Sulfur has six valence electrons. Valence electrons are the outermost electrons which, therefore, are located on the highest energy levels.
Why does boron only need 6 valence electrons?
Boron has a charge of 5. This is balanced by 5 electrons. The valence electrons may participate in bonding through sharing with other atoms, to make three bonds. Three bonds = six electrons.
Why can phosphorus make 5 bonds?
Phosphorus can have expanded octet, because it can shift it’s lone pair electrons (3s orbital electrons) to empty 3d obital during excited state and thus can form 5 bonds.
Why can an atom only have 8 valence electrons?
The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. When atoms have fewer than eight electrons, they tend to react and form more stable compounds.
Why does the third shell have 8 electrons?
Sulfur Trioxide Number Of Electrons
The electron capacity of the third shell is 8, when there are no shells above it. And that is the case for all elements in the third period. It is only when there are outer shells surrounding it that the third (or higher) shell has a higher capacity.
Why is the maximum number of valence electrons 8?
Sulfur Number Of Electrons To Gain
Generally, the maximum number of electrons that the outermost shell of an atom can have is 8 (eight). This is called an octet and the restriction is because the valence electrons are generally from the s and p orbitals which can have a maximum of 8 electrons.
What is the charge for sulfur?
1.17: Ions
| Element | Protons | Net Charge |
|---|---|---|
| Potassium atom | 19 | |
| Potassium ion | 19 | +1 |
| Sulfur atom | 16 | |
| Sulfur ion | 16 | −2 |
How do you find the Valency of Sulphur in SO2?
Sulphur has the atomic number of 16. Its electronic configuration is 2,8,6. The valency of S depends upon its oxidation state and with which element it reacts. For example, the valency of S in SO2 is +4 and in SO3 is +6.
Does sulfur have 3 valence electrons?
Sulfur Number Of Electrons In Outer Shell
Valence electrons are the electrons in the outermost principal quantum level of an atom. Sometimes, the outermost energy level is called the valence shell. The outer energy level in this atom is n = 3. It holds six electrons, so sulfur has six valence electrons.
How many bonds can sulfur form?
Sulfur, like oxygen, frequently forms two bonds.
Sulfur Number Of Electrons To Fill Outer Shell
Does sulfur electron dot diagram have six dots?
Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom.
